| Cancer Information Highlights | | From the National Cancer Institute | | Updating you about cancer causes, prevention, screening, treatment, coping, and more | | | | New from NCI | | What Causes Immunotherapy's Heart-Related Side Effects? | 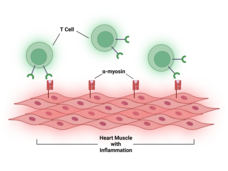 | | Myocarditis, or inflammation in the heart, is a rare but often fatal side effect of treatment with immune checkpoint inhibitors. Researchers have now found a potential chief cause of this problem: T cells attacking a protein in heart cells called alpha-myosin. | | Chemo Drug Redesigned to Avoid Side Effects | 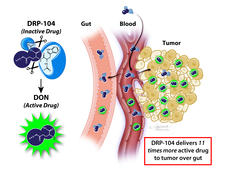 | | Researchers have changed a chemo drug, once abandoned because it caused serious side effects in the gut, so that the drug is only triggered in tumors. After promising results in mice, the drug, DRP-104, is now being tested in a clinical trial. | | Advances in Ovarian Cancer Research |  | | This new page highlights recent advances in research to prevent and treat cancer that starts in the ovaries, fallopian tubes, and the membrane that lines the abdominal cavity (also called the peritoneum). | Targeted Therapy Approved for High-Risk Hodgkin Lymphoma in Young People
Based on an NCI-sponsored clinical trial conducted by the Children's Oncology Group, the Food and Drug Administration (FDA) has approved the drug brentuximab vedotin (Adcetris) in combination with chemotherapy for some young people with Hodgkin lymphoma. | Monoclonal Antibody Approved for Advanced Alveolar Soft Part Sarcoma
A clinical trial led by NCI has resulted in the first approval of a treatment for advanced alveolar soft part sarcoma (ASPS). FDA recently approved the immunotherapy drug atezolizumab (Tecentriq) for the treatment of some adults and children with ASPS.
| | FDA Approvals | Mirvetuximab Soravtansine-gynx
We've added a drug summary for mirvetuximab soravtansine-gynx (Elahere). FDA recently approved this drug to treat ovarian cancer, fallopian tube cancer, or primary peritoneal cancer that is folate receptor–alpha positive. It is used in adults whose cancer did not respond to or is no longer responding to platinum chemotherapy and who have received one to three types of systemic therapy. | | | | | | Also of Interest | Eating Hints: Before, during, and after Cancer Treatment
This updated booklet describes ways to eat well before, during, and after treatment for cancer. It can help you understand eating problems that may happen and suggests ways to deal with them. | COVID-19: What People with Cancer Should Know
COVID-19 rates are rising. Read the latest on how to protect yourself, including new information from the Centers for Disease Control and Prevention (CDC) about COVID vaccines that target the Omicron variant for children as young as 6 months old. You'll also find information about treatment for people who are more likely to get very sick from COVID-19 and special considerations for people with cancer. | Contact Us for Help
Information specialists at NCI's Cancer Information Service (CIS), NCI's contact center, are available to help answer your cancer-related questions in English and Spanish. Reach us by phone, chat, or email. | | | | |
No comments:
Post a Comment